RR6 Calculator
The RR6 model predicts survival in myelofibrosis based on clinical response after 6 months of ruxolitinib.
Reference
Maffioli M, Mora B, Ball S, Iurlo A, Elli EM, Finazzi MC, Polverelli N, Rumi E, Caramella M, Carraro MC, D'Adda M, Molteni A, Sissa C, Lunghi F, Vismara A, Ubezio M, Guidetti A, Caberlon S, Anghilieri M, Komrokji RS, Cattaneo D, Della Porta MG, Giorgino T, Bertù L, Brociner M, Kuykendall AT, Passamonti F. A Prognostic Model to Predict Survival After 6 Months of Ruxolitinib in Patients with Myelofibrosis. Blood Advances 2022. bloodadvances.2021006889. doi:10.1182/bloodadvances.2021006889
Data at baseline
Evaluation at ruxolitinib startData at 3 months
Evaluation 3 months after ruxolitinib startData at 6 months
Evaluation 6 months after ruxolitinib startRR6 model results
Risk stratum: -
Median overall survival*: months
Median o. survival* 95% CI: months
*counted from 6 months post ruxolitinib start.Calculation
Risk points:- spleen length reduction ≤30%:
- low ruxolitinib dose:
- transfusion status:
Total risk points:
Legend
LCM - left costal marginBID - bis in die, twice daily
RBC - red blood cell
Survival curves
Actuarial survival curves of the 3 risk groups of patients according to the Response to Ruxolitinib after 6 months (RR6) developed in ruxolitinib-treated myelofibrosis patients (training cohort).
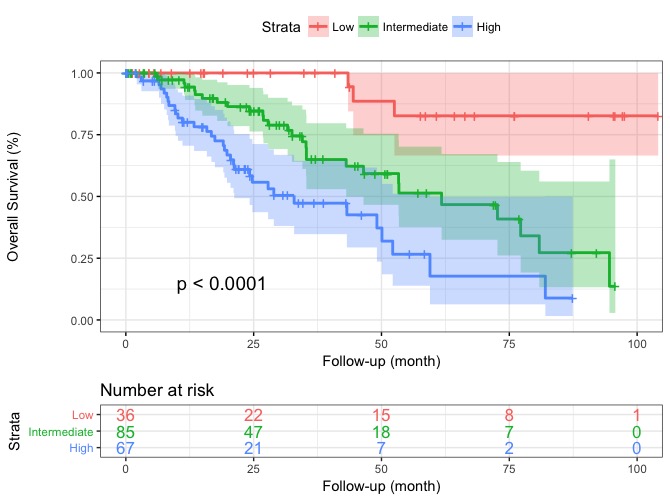
Source: Maffioli et al., A Prognostic Model to Predict Survival After 6 Months of Ruxolitinib in Patients with Myelofibrosis. Blood Advances. doi:10.1182/bloodadvances.2021006889